
If you peek into the first-aid kit of an experienced hiker, you’ll find the usual suspects: bandages, blister tape, and antiseptic wipes. But nestled among them, you’ll often spot a small, familiar blue jar of cold rub. While it’s a classic for battling winter colds, on the trail, it’s valued for a completely different set of reasons.
Savvy hikers know this ointment isn’t just for stuffy noses; it’s a versatile multi-tool for tackling two of the most common trail nuisances: bugs and sore muscles.
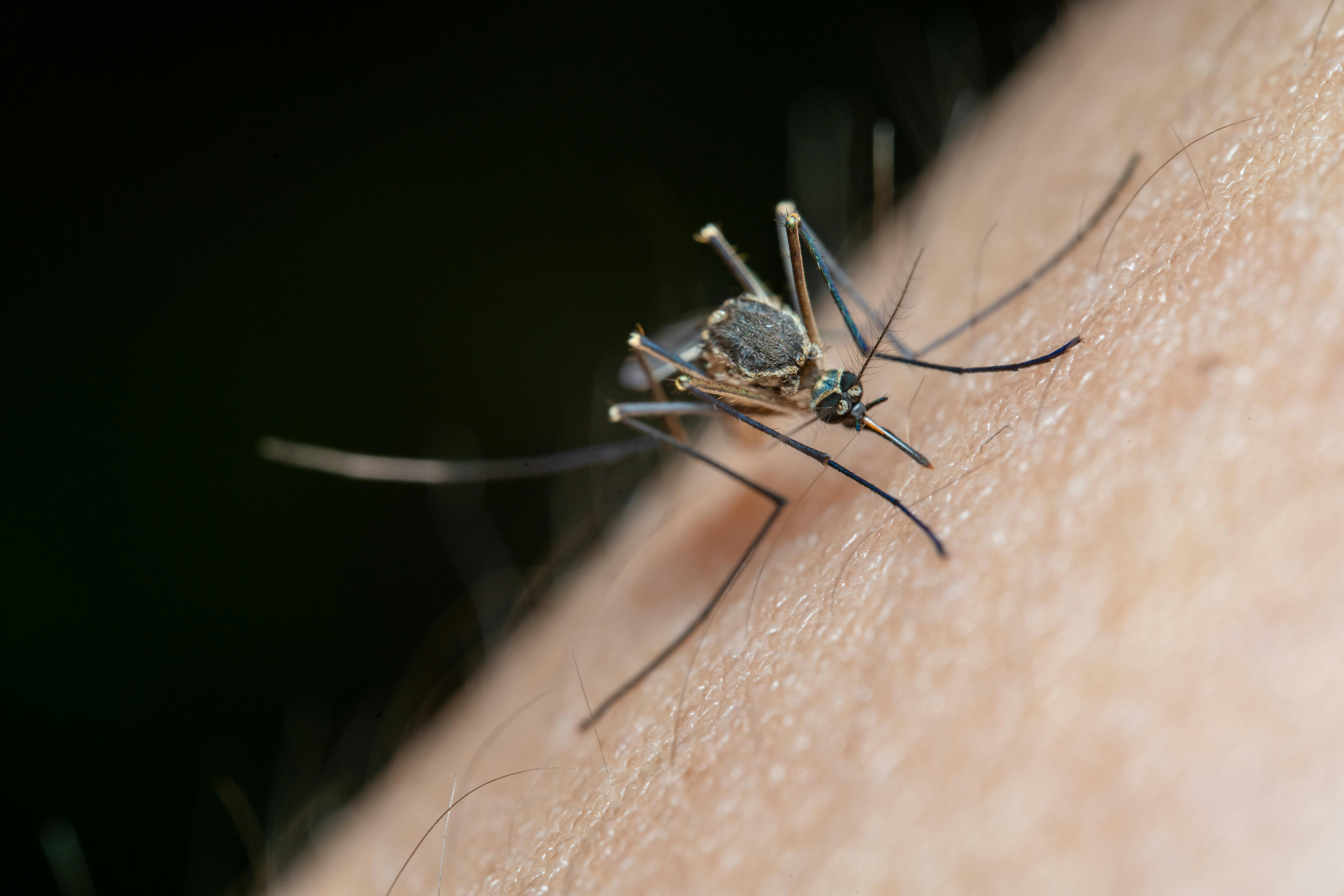
1. The Unlikely Insect Repellent
Why would someone rub a medicated chest ointment on their skin to keep bugs away? The answer is in the smell.
- The Power of the Punchy Aroma: Most insects, especially mosquitoes, navigate the world largely through scent. They are attracted to the carbon dioxide we exhale and our body odor.
- An Overpowering Scent Mask: Cold rub is packed with potent-smelling ingredients like menthol, camphor, and eucalyptus oil. When applied to pulse points (like wrists, ankles, and neck), it creates a powerful aromatic barrier. The strong medicinal smell can help mask the natural human scents that draw bugs in, making you less of a target.
- A Note of Caution: It’s important to know that while many hikers swear by this hack, it is not a registered insect repellent. Its effectiveness can vary, and it may need more frequent reapplication. However, in a pinch, it’s a better-than-nothing solution that most already have on hand.

2. The Soothing Relief for Trail-Weary Muscles
After a long day with a heavy pack, every hiker knows the feeling of aching quads, tight calves, and sore shoulders. This is where the cold rub truly shines.
- The Sensation of Relief: The key ingredient here is menthol. When applied to the skin, menthol doesn’t actually change the temperature of your muscles. Instead, it brilliantly tricks your brain. It stimulates the nerve receptors that sense cold, creating a powerful cooling sensation. At the same time, ingredients like camphor provide a mild warming feeling.
- Distracting the Mind: This dual-sensation of hot and cold acts as a “counter-irritant.” It provides a strong, new sensation that effectively distracts your brain from the deeper, dull ache of your tired muscles. While it doesn’t heal the muscle fatigue itself, it provides significant temporary comfort, making it easier to relax and recover at camp.
The Ultimate Multi-Tool for Your Pack
For a hiker, every ounce matters. A product that can solve multiple problems is worth its weight in gold. A single jar of cold rub can:
- Deter buzzing insects on the trail.
- Soothe sore muscles at the end of the day.
- And, of course, still be used for its original purpose: clearing sinuses if you catch a chill from a cold night or sudden weather change.
It’s this trifecta of benefits that secures the cold rub’s place as a trusted, off-label staple in the wilderness. It’s a little jar of comfort, proving that sometimes the most valuable gear isn’t the most high-tech—it’s the simplest, most versatile tool you can find.





















